Taming the Acid Beast: The Baking Soda Solution
Imagine a volatile substance, a potent force capable of corroding metal and inflicting severe burns. Now, picture a humble kitchen staple, a white powder often associated with baking and cleaning. What happens when these two worlds collide? The reaction between sulfuric acid and baking soda is a compelling example of acid-base chemistry, a process that transforms a hazardous material into something far less harmful.
Sulfuric acid, a strong mineral acid, is widely used in various industrial processes. Its highly corrosive nature makes it a crucial component in battery manufacturing, fertilizer production, and metal processing. However, this powerful acid also presents significant safety risks. Accidental spills or improper handling can lead to serious consequences. Fortunately, a readily available substance can effectively counteract sulfuric acid's corrosive power: baking soda, also known as sodium bicarbonate.
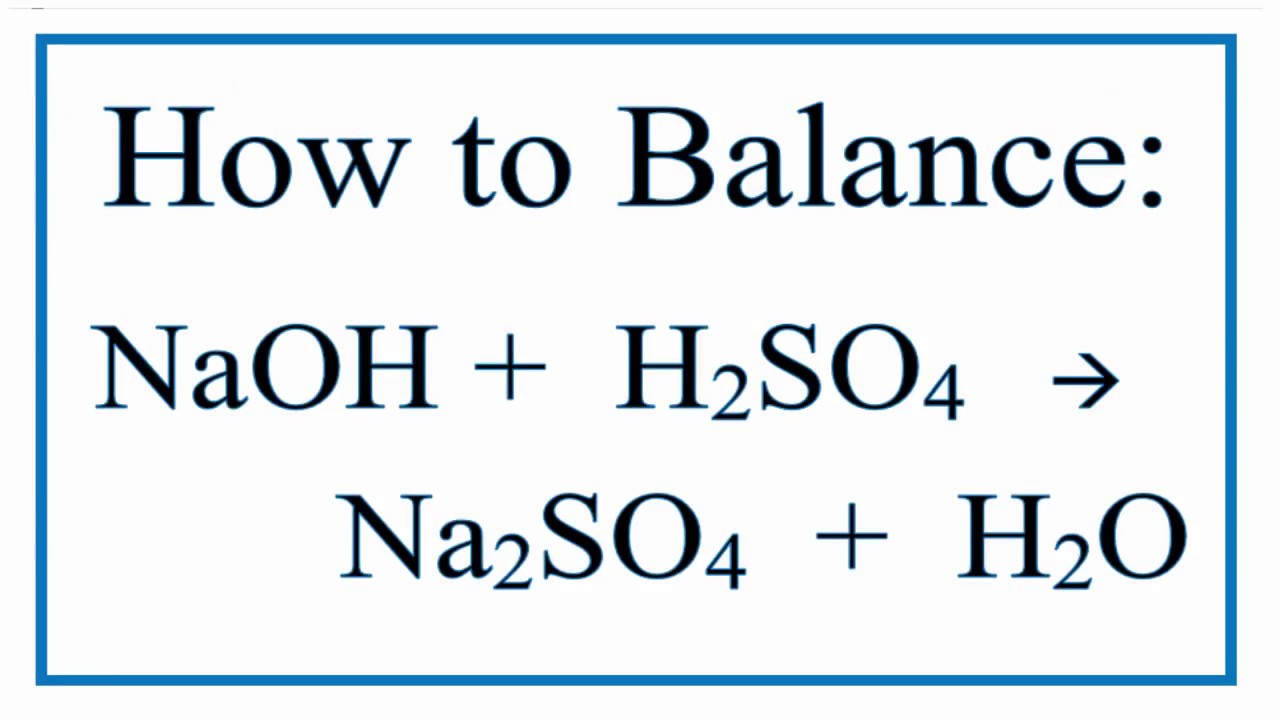
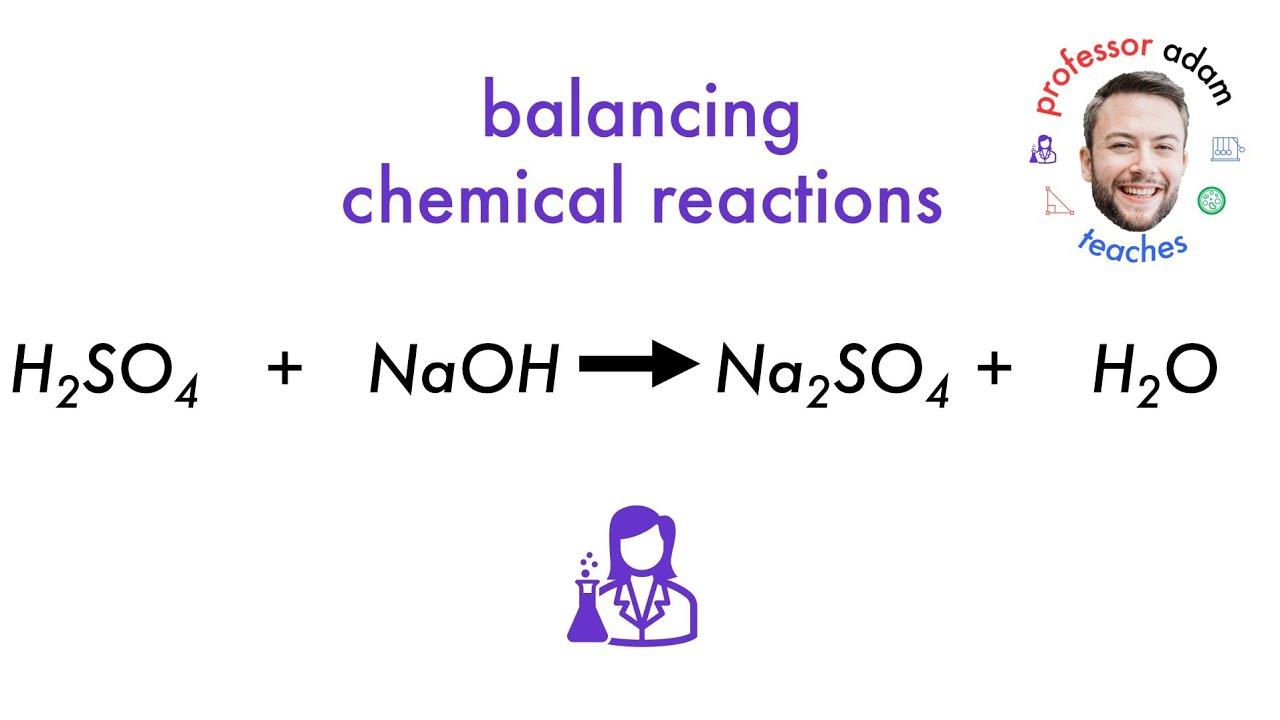
The chemical reaction between sulfuric acid and baking soda is a neutralization reaction. In this process, the baking soda acts as a base, accepting protons from the sulfuric acid. This interaction produces sodium sulfate, carbon dioxide, and water. The release of carbon dioxide is often observed as fizzing or bubbling, a visual cue that the neutralization process is underway.
Neutralizing sulfuric acid requires careful attention to safety. The reaction can be exothermic, meaning it releases heat. Furthermore, the generated carbon dioxide gas can build up pressure, especially in a closed container. Understanding these potential hazards is crucial for safe and effective neutralization. Always add the baking soda slowly to the acid, never the other way around, to control the reaction and prevent violent bubbling or splashing.
The practice of using common household substances to neutralize strong acids has likely existed for centuries, though formal documentation is scarce. Early chemists and alchemists experimented with various materials to understand their properties and interactions. While specific historical accounts of neutralizing sulfuric acid with baking soda are difficult to pinpoint, the underlying chemical principles have been understood and applied for a long time.
One of the key benefits of using baking soda to neutralize sulfuric acid is its accessibility. Baking soda is a readily available and inexpensive household item. This makes it a practical choice for dealing with small acid spills or leaks. Additionally, the byproducts of the reaction—sodium sulfate, carbon dioxide, and water—are relatively benign compared to the original sulfuric acid.
Another advantage is the ease of use. Simply sprinkling baking soda onto the spilled acid and observing the reaction is relatively straightforward, although caution should always be exercised. The fizzing action provides a visual confirmation that the neutralization process is occurring.
A third benefit is the relatively low environmental impact of the byproducts. Sodium sulfate is a common ingredient in various products, and carbon dioxide, while a greenhouse gas, is produced in relatively small quantities during this reaction.
Step-by-step guide to neutralizing sulfuric acid with baking soda:
1. Wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat or apron.
2. Slowly sprinkle baking soda onto the spilled sulfuric acid.
3. Observe the fizzing reaction. Continue adding baking soda until the fizzing stops.
4. Once the reaction has subsided, carefully dispose of the neutralized mixture according to local regulations.
Advantages and Disadvantages
| Advantages | Disadvantages |
|---|---|
| Readily available | Not suitable for large spills |
| Inexpensive | Reaction can be exothermic |
| Easy to use | Produces carbon dioxide gas |
Best Practices:
1. Always wear PPE.
2. Add baking soda slowly.
3. Ensure good ventilation.
4. Dispose of waste properly.
5. Never add water to acid.
Frequently Asked Questions
1. Is it safe to neutralize sulfuric acid with baking soda? Yes, but precautions are necessary.
2. What are the byproducts of the reaction? Sodium sulfate, carbon dioxide, and water.
3. What should I do if I spill a large amount of sulfuric acid? Contact emergency services.
4. Can I use other substances to neutralize sulfuric acid? Yes, but baking soda is a common and accessible option.
5. What should I do with the neutralized mixture? Dispose of it according to local regulations.
6. Is the reaction exothermic? Yes, it releases heat.
7. What happens if I add water to sulfuric acid? It can cause a violent reaction.
8. Where can I learn more about acid-base reactions? Chemistry textbooks or online resources.
Tips and Tricks
Practice safety first. Always use small amounts of baking soda initially and observe the reaction before proceeding. Ensure adequate ventilation to prevent the buildup of carbon dioxide.
Neutralizing sulfuric acid with baking soda is a powerful demonstration of basic chemistry principles at work. This readily available household item provides an effective and accessible way to counteract the corrosive power of a strong acid. Understanding the process, adhering to safety precautions, and following best practices empowers individuals to handle minor acid incidents responsibly. The ability to tame a potent substance like sulfuric acid with a common kitchen staple underscores the importance of understanding chemical reactions and their practical applications in everyday life. By carefully following the guidelines outlined above, you can safely and effectively neutralize sulfuric acid using baking soda, turning a potentially dangerous situation into a manageable one. Always prioritize safety and remember that for large spills or complex scenarios, professional assistance is crucial.
Mastering your boat lift motor wiring
Finding stillness exploring the interplay of living and non living things
Imagining the future the power of sci fi warship concept art